Making Solutions in the Laboratory
6/3/2014
JoVE Science Education Database. General Laboratory Techniques. Making Solutions in the Laboratory. Journal of Visualized Experiments, Cambridge, MA, doi: 10.3791/5030 (2014).
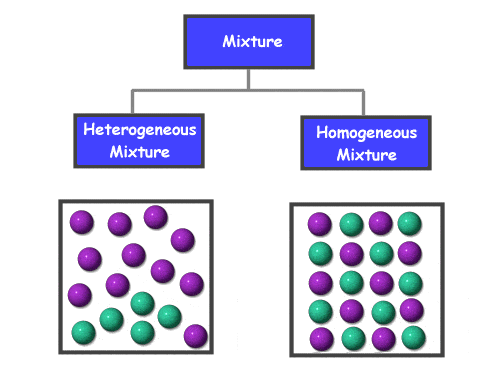
The ability to successfully make solutions is a basic laboratory skill performed in virtually all biological and chemical experiments. A solution is a homogenous mixture of solute dissolved in bulk liquid known as the solvent. Solutions can be described by their solute concentration, a measure of how much solute is present per unit of solution. In this video, a step-by-step procedure for how to make a water-based, or aqueous, solution for biological applications is presented. The video discusses how to calculate and measure the amount of solute needed for a given volume of solution. Methods for dissolving the solute in purified water and adjusting the pH of the solution are shown. Proper addition of the quantity sufficient (QS) to reach the desired volume is demonstrated with respect to the meniscus before discussing methods for sterilizing the solution. Applications of making solutions are presented through the discussion of several commonly used biological solutions, such as phosphate buffered saline (PBS), and their uses in biological research. These solutions are buffers that mimic physiological pH and osmolarity of cellular fluids.