Determining the Activity of Mucosal Adjuvants
6/3/2014
Baudner BC, Giudice GD. Methods Mol Biol. 2010;626:261-85. doi: 10.1007/978-1-60761-585-9_18.
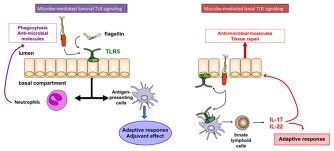
Mucosal vaccination offers the advantage of blocking pathogens at the portal of entry, improving patient’s compliance, facilitating vaccine delivery, and decreasing the risk of unwanted spread of infectious agents via contaminated syringes.Recent advances in vaccinology have created an array of vaccine constructs that can be delivered to mucosal surfaces of the respiratory, gastrointestinal, and genitourinary tracts using intranasal, oral, and vaginal routes. Due to the different characteristics of mucosal immune response, as compared with systemic response, mucosal immunization requires particular methods of antigen presentation. Well-tolerated adjuvants that enhance the efficacy of such vaccines will play an important role in mucosal immunization. Among promising mucosal adjuvants, mutants of cholera toxin and the closely related heat-labile enterotoxin (LT) of enterotoxigenic Escherichia coli present powerful tools, augmenting the local and systemic serum antibody response to co-administered antigens.In this chapter, we describe the formulation and application of vaccines using the genetically modified LTK63 mutant as a prototype of the family of these mucosal adjuvants and the tools to determine its activity in the mouse model.